Fs time domain spectroscopy on Peroxides
In combustion processes, alkylperoxyl radicals (ROO·) are formed by rapid addition of alkyl radicals to oxygen. In order to understand the chemistry of alkyl peroxyl radicals, it is essential to have an accurate grasp of the spectroscopy and dynamics of these species. In this project we aim to apply intense ultrafast fs laser pulses to produce and to investigate alkylperoxyl radicals by means of time resolved four wave mixing (FWM) methods. The application of ultra short laser pulses provides the ability to produce alkylperoxy (RO·) and alkylperoxyl (ROO·) radicals by direct photo dissociation of particular dialkylperoxides (ROOR) at excitation wavelengths around 200 nm.
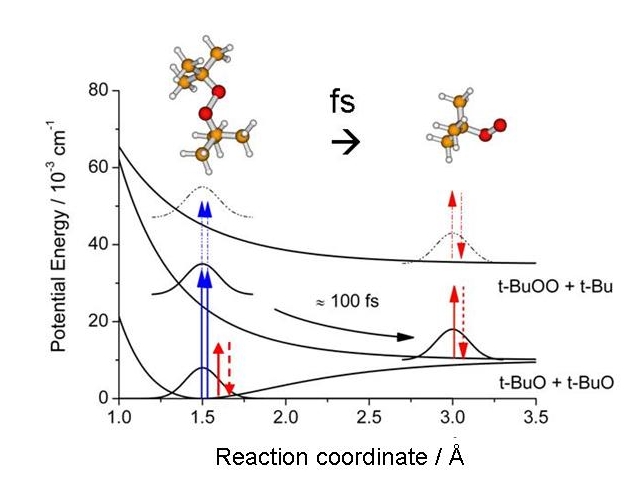 |
Figure: Potential energy surfaces and TG excitation scheme of di-tertbutyl peroxide.
This early time response is mainly characterized by a time-zero coherence spike followed by a rotational feature, which is caused by a fast dephasing of the initially excited rotations. From such early time signals it is possible to determine, even in the absence of rotational recurrences, the main rotational constants (A,B,C) of a molecule with a two-digit accuracy. Hence, in some cases the early time response can be a sufficient indication to differentiate between precursor molecules, alkylperoxyls and further dissociation products.
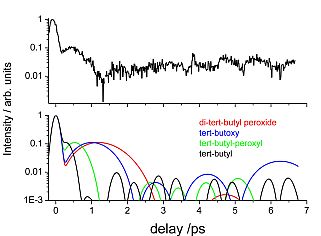 |
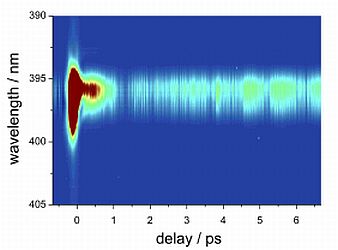 |
Figure: Logarithmic plot of frequency integrated UV resonant fs-TG signal compared to simulations of the different probable dissociation products. The corresponding frequency dispersed signal is depicted on the right.