Fs mass spectrometry and photoionization
Time-of-flight mass spectrometry (Tof-MS) is a well established technique for determining
ions masses and their relative concentrations. Tof-MS begins with gas-phase ion formation and
then involves ion acceleration through an electric field into a field-free drift region. The acceleration voltage, giving all
ions the same kinetic energy, propels the ions at different velocities based on their specific
mass-to-charge ratios. Subsequently, the ions are allowed to drift in a field free region where
they separate spatially as a function of their individual kinetic energies. Lighter ions will
move faster than heavier ions. Following ion detection, peaks representing ion-packets each having the same mass comprise the
mass spectrum, and the time needed to travel the length of the drift region can be related to the mass of the ion.
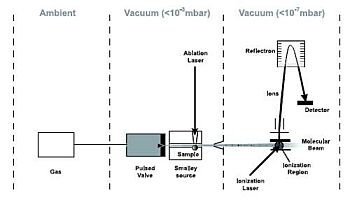 |
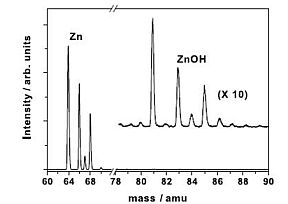 |
Figures: Time of flight fs multiphoton ionization setup and a corresponding ion mass spectrum from an Zn ablation source.
The source can be exchanged by either a pyrolytic, a photolytic or a discharge source.
In the context of renewable energy storage processes,
the Zn/ZnO redox-cycle gained much attention in the
last years. The aim is to store solar thermal energy in
the form of metallic zinc by dissociation of zinc oxide in
a solar heated reactor. The process inside the reactor
comprises in essence two steps: evaporation and
dissociation of solid ZnO(s),
ZnO(s) --> Zn(g) + O
and subsequent rapid cooling with condensation of zinc,
Zn(g) --> Zn(s). Due to re-oxidation, possibly
accelerated already in the gas phase by the presence of
emanating zinc oxide clusters, the efficiency of the overall
energy storage yield may be rather poor unless adequately
rapid quenching of zinc can be realized.
Neutral ZnO and ZnOH molecules could be produced in a molecular beam by expansion of laser ablated zinc together with H2O,
O2 or N2O seeded in a rare gas (Ar, Ne, He). Due to the characteristic Zn isotope distribution, the zinc containing compounds, ionized with a 100 fs
laser pulse, could unambiguously be identified with a TOF mass spectrometer. The abundance of ZnOH produced in our experiments exceeds
the one of ZnO and ZnN by orders of magnitude if H2O is present in the system. Small quantities of (ZnO)2H and Zn2(OH)3 compounds could
also be observed. To our knowledge this is the first evidence for the occurrence of neutral ZnO and ZnOH molecules in a molecular beam.
D. Cannavo, G. Knopp, P. Radi, P. Beaud, M. Tulej, P. Bodek, T. Gerber, and A. Wokaun, Journal of Molecular Structure, 782, 67-72 (2006).
Back
.
