
Updated:
20.12.2017




|
 |

| Laser Vaporization Source
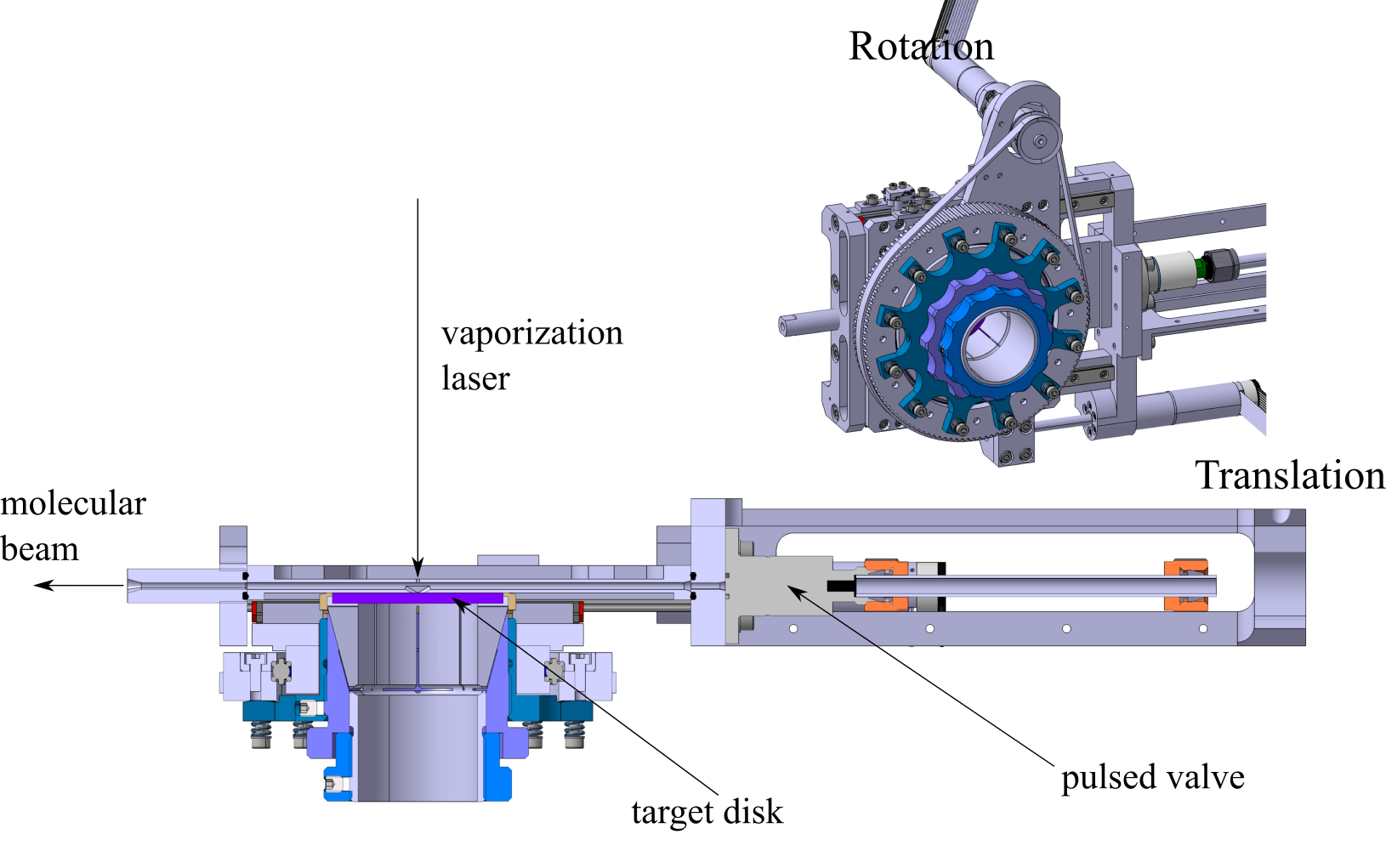
A schematic of the laser vaporization source is shown in the figure. In this design, the vaporization laser
enters the source perpendicular to the direction of expansion and
is focused onto the metal target (Cu 99% purity). The target is
translated and rotated by a pair of electric motors (Maxon). The
motors allow careful control of the rate at which fresh metal sur-
face is sampled. The target disk is sealed against the cluster
source body by a polyetheretherketone (PEEK) o-ring. To ensure
that the target surface remains parallel to the source body, the
target mount is mechanically decoupled from the axis of rotation
by a set of springs. The second harmonic (532 nm) output of a
Nd:YAG laser (Continuum, NY81) is used as a vaporization.
|
 |
| Pyrolytic
Generation of Radicals
A pyrolytic
source has been build following the original design of Prof. Peter Chen,
ETH Zurich. A neon gas pulse with an appropriately designed organic precursor
is expanded through a silicon carbide tube that is resistively heated
to 1800 C. For example, the production of the CH3 radical from
CH3CN has been demonstrated by using femtosecond ionization mass spectrometry.
|
 |
| Photolytic
Generation of Radicals
Laser-induced
fluorescence spectroscopy of the ketenyl radical has been performed in
a colaboration with the Sandia National Laboratories, Livermore, California.
HCCO has been produced by the photolysis of ketene, CH2CO, at 193 nm in
the early portion of a free jet expansion. The photolysis yield at 193
nm for H+HCCO is ~37% [1].
Degenerate four-wave mixing of OH in the molecular beam has been observed
in our labs. OH radicals are produced by photolysis of t-butyl-hydroperoxide,
(CH3)3COOH, at 248 nm.
[1] L.R. Brock, B. Mischler, and E.A. Rohlfing, "Laser-induced fluorescence spectroscopy of the B-tilde [sup 2] Pi--X-tilde [sup 2]A[sup [double-prime]] band system of HCCO and DCCO", The Journal of Chemical Physics, vol. 110, Apr. 1999, pp. 6773-6781. |
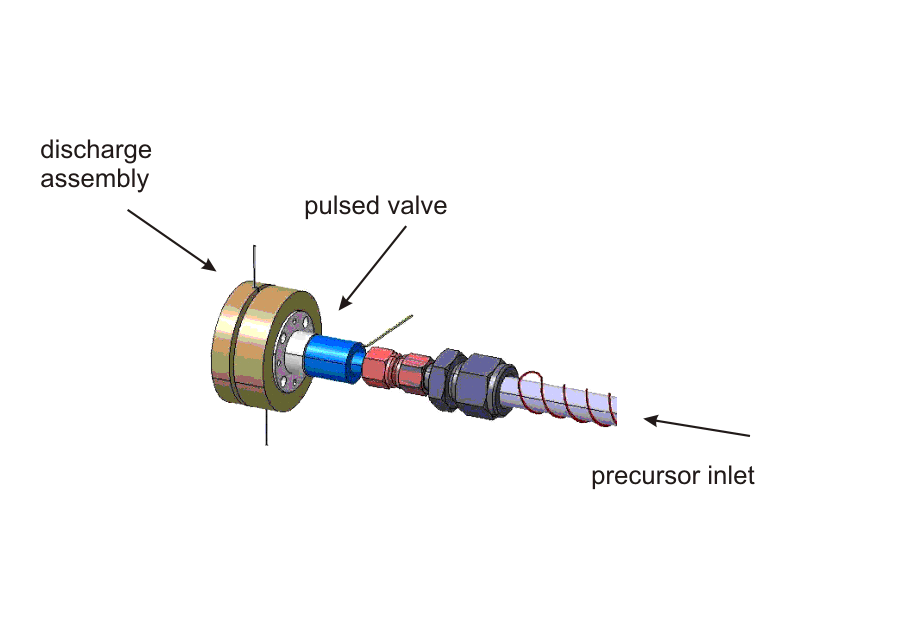
| Pin-hole Discharge Source |
 |
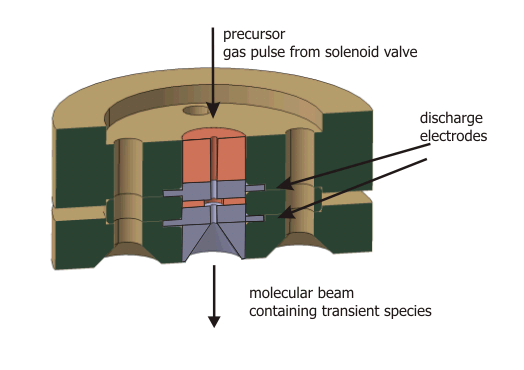 |
| The discharge assembly containing a set of electrically isolated stainless steel disc electrodes mounted on the valve body (general valve, PARKER) by glass ceramic spaces (MACOR). A typical experimental procedure starts with a trigger to the solenoid valve to expand a precursor diluted in a rare gas (for example C2H2 in Ar). At a variable time after the initial trigger for the valve, a high-voltage pulse of ≈ 800 V and adjustable duration is applied to the discharge electrodes. By changing the polaritiy of the discharge, the direction of the electrons to either co- or counter-propagation with respect to the molecular beam can be chosen. A careful optimization of the voltage, trigger delay, trigger duration an polarity yields an intense and remarkably stable molecular beam.
|
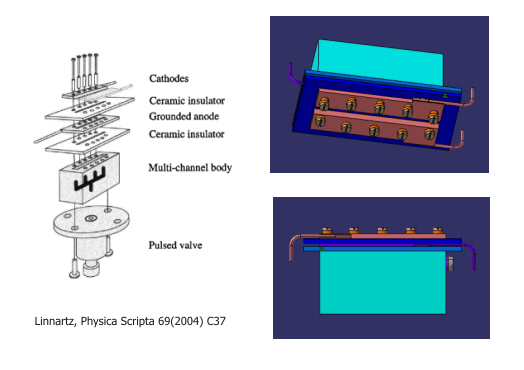
| Slit Discharge Source
Discharge assembly containing a set of electrically isolated steel electrodes mounted on a multi-channel body by glass ceramic spacers. The planar, two-dimensional expansion through a long and narrow slit offers an essentially Doppler-free environment. The higher density of the radicals promotes equilibration of the translational and internal degrees of freedom. In addition, the two-dimensional geometry is optimally suited for a boxcars configuration of the four-wave mixing experiments.
|
 |
Back
|
 |
